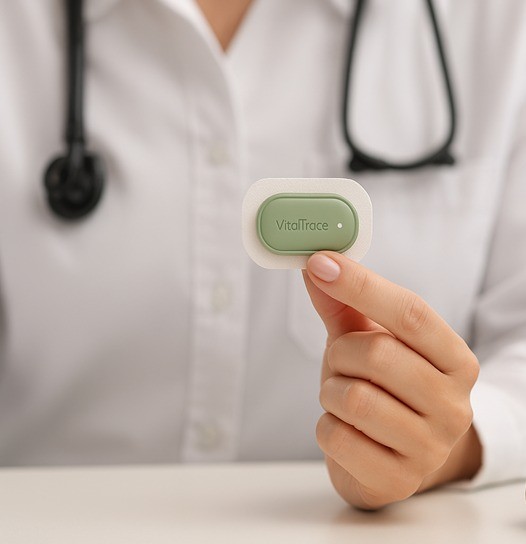
Pioneering continuous lactate monitoring - the future of healthcare in childbirth and beyond
Pioneering continuous lactate monitoring - the future of healthcare in childbirth and beyond
Childbirth is still unsafe for mothers and babies even in the most advanced health care systems
The day we are born is the most dangerous day of our lives .
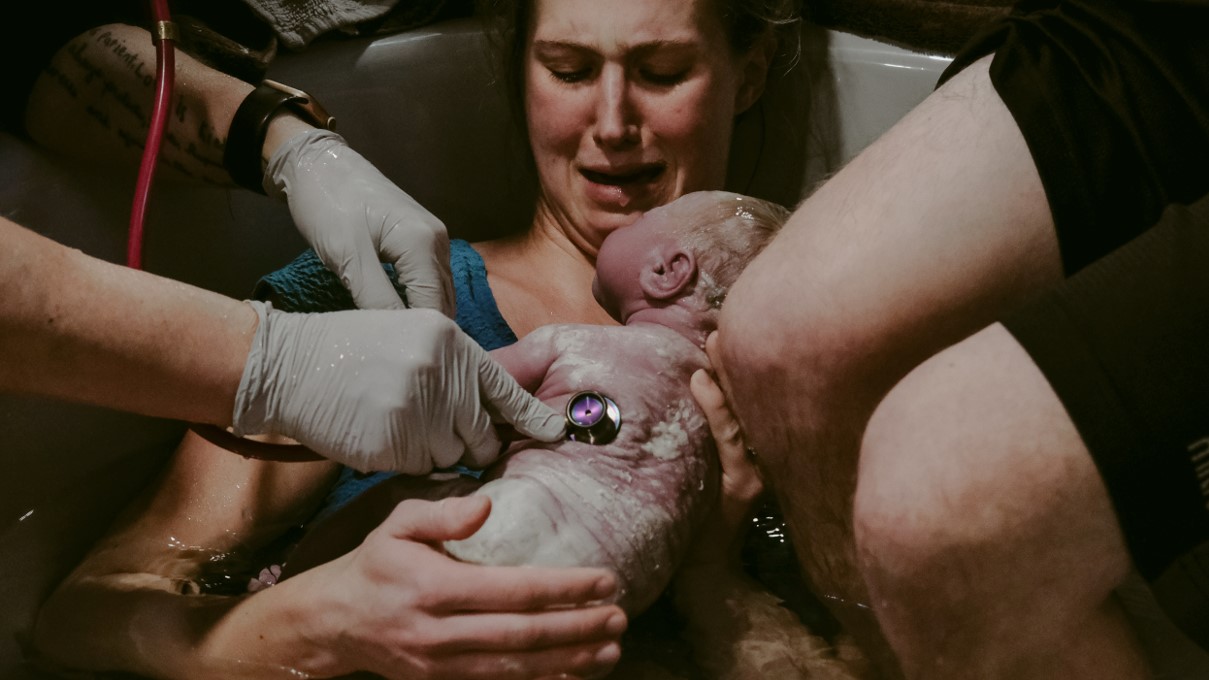
Obstetricians currently lack accurate and continuous, validated information to make evidence-based decisions when managing childbirth.
DelivAssure, the world’s first continuous lactate biosensor for childbirth monitoring
Continuous lactate monitoring would allow clinicians to make evidence-based decisions in childbirth.
FDA Breakthrough Device Status
DelivAssure is a clinical-stage product granted Breakthrough Device Designation by the FDA.
Designed for Comfort and Care
Built to support maternal movement, DelivAssure features a minimal, unobtrusive design for both mothers and midwives.
Objective Data to Save Lives
DelivAssure is the first to continuously detect a direct marker of fetal distress—enabling timely, life-saving decisions during childbirth.
Designed for Clinical Adoption
A familiar form factor with intuitive workflow integration to maximize adoption from day one.
Breakthrough sensor technology unlocking continuous lactate in real-time
DelivAssure can be the first solution to continuously monitor objective and clinically validated biomarkers.
Breakthrough sensor technology unlocking continuous lactate in real-time
DelivAssure can be the first solution to continuously monitor objective and clinically validated biomarkers.
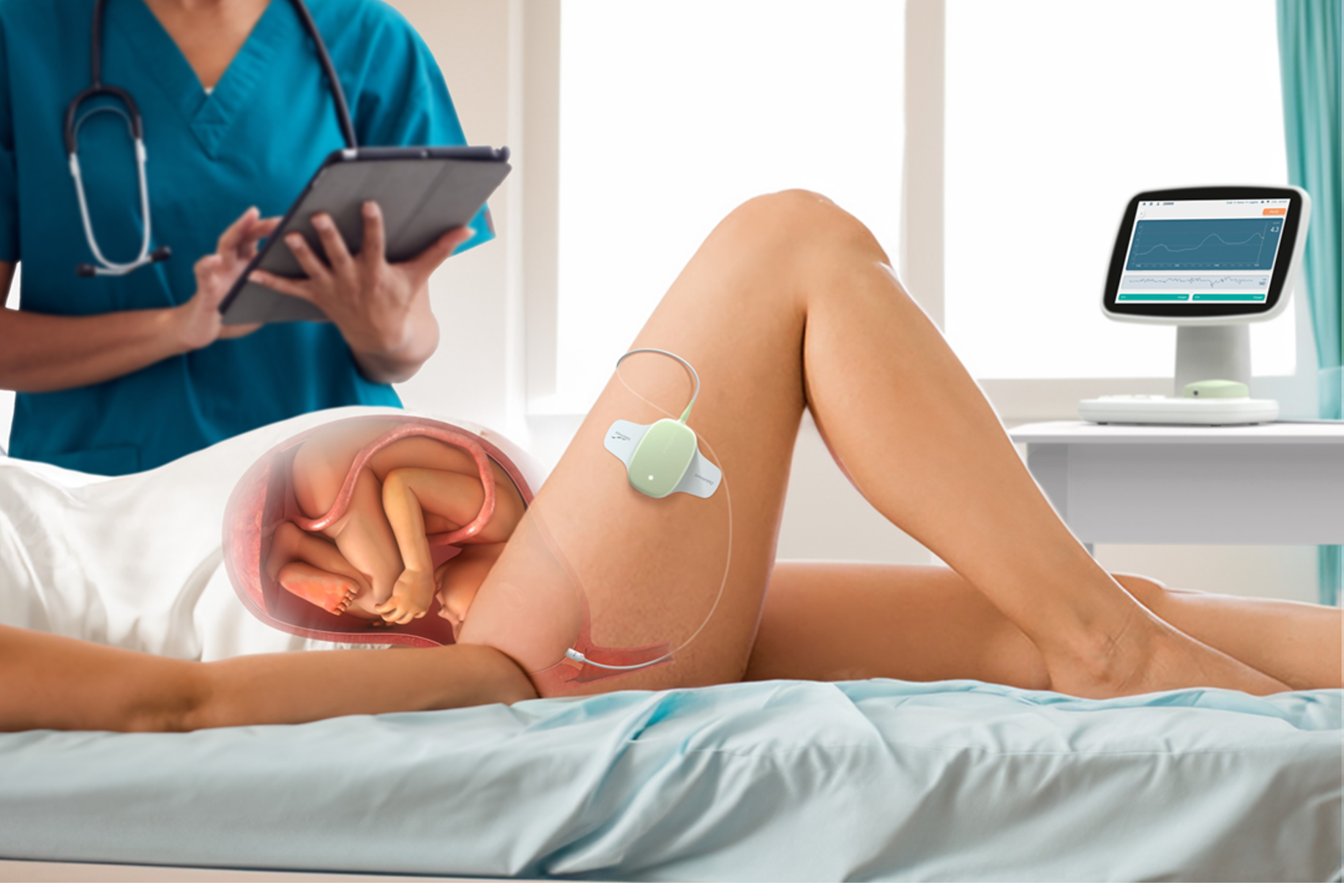
Our proprietary chemistry is layered onto a minimally invasive macroneedle sensor, engineered for ease of insertion during labour.

VitalTrace utilises enzymatic electrochemical sensors—similar to continuous glucose monitors—that interface with interstitial fluid to detect lactate, the gold-standard indicator of life-threatening fetal hypoxia.

Alternative technologies like ST-analysis and fetal pulse oximetry have been trialled extensively and have failed to become the worldwide solution.
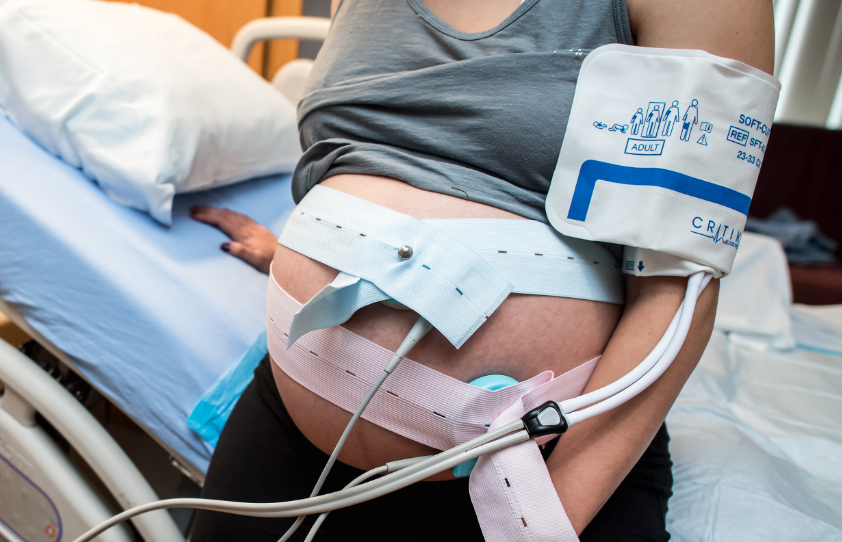
Fetal heart rate monitoring, the current standard of care, is an indirect and often inaccurate proxy for oxygen deprivation and acidosis.
Hear from the obstetricians
DelivAssure can be the first solution to continuously monitor objective and clinically validated biomarkers.
Our Latest News
The latest breakthroughs, milestones, and updates from the world of VitalTrace — all in one place.

VitalTrace awarded $500K to expand platform technology for neonatal intensive care
VitalTrace has been awarded $500,000 as part of the Government of Western Australia’s Future Health Research and Innovation (FHRI) Fund’s Innovative Solutions – Precision Health program.
We are proud to share that VitalTrace has been awarded $500,000 as part of the Government of Western Australia’s Future Health Research and Innovation (FHRI) Fund’s Innovative Solutions – Precision Health program.
VitalTrace is poised to revolutionise childbirth with DelivAssure, following our world-first clinical trial (read about it here.
This recent grant win allows us to extend our impact beyond childbirth and into a baby’s first days of life. Our continuous lactate monitoring (CLM) technology will be adapted to detect elevated lactate in neonates, an early indicator of neonatal sepsis, which has a mortality rate of 17-25%.
Lactate is a vital biomarker of metabolic stress, rising in not only sepsis, but other serious complications such as congenital heart disease and necrotising enterocolitis.
With our CLM technology, clinicians could gain real-time and accurate insights into neonatal wellbeing without repeated heel pricks or blood draws, facilitating early diagnosis, better management and reducing pain, trauma and blood loss in critically ill newborns.
Thank you to the FHRI for supporting our mission to significantly improve newborn outcomes and drive precision health innovation in Western Australia.
See the announcement here.

VitalTrace's DelivAssure featured on national news as a potential breakthrough
Our continuous lactate monitor for childbirth, DelivAssure, has been featured on a number of media platforms, including the ABC, as a potential breakthrough.
A breakthrough in childbirth care and a proud moment for the VitalTrace team. Our continuous lactate monitor for childbirth, DelivAssure, has been featured on a number of media platforms, including the ABC, as a potential breakthrough.
A moment of thanks on the achievement of the recent clinical trials:
We want to especially thank our Chief Medical Officer Jonathan Morris, the tireless Sharon McCracken, and our long-time collaborator Jane Pillow, who have been integral to our progress.
Thank you to our Principal Investigators across our clinical trials: A/Professor Sean Seeho, Penny Sheehan, Daniel Rolnik and Scott White. We’re incredibly grateful to the clinical teams and hospitals who made these trials possible across Royal North Shore Hospital, Monash Health, King Edward Memorial Hospital and Box Hill Hospital - Eastern Health.
The mothers and babies who took part in our trials are also at the heart of our work. We are endlessly appreciative for their participation.
Also with thanks to our partners and supporters: The University of Western Australia, University of Sydney, University of Technology Sydney, Australian Government and Government of Western Australia's Future Health Research and Innovation (FHRI) and Department of Jobs, Tourism, Science and Innovation (JTSI), Fund WA and Artesian (Alternative Investments).
Most of all, a huge thank you to our entire VitalTrace team whose hard work and dedication everyday have lead to this outcome.

VitalTrace's first peer-reviewed journal article published in The SMFM Pregnancy Journal
Our first peer-reviewed journal article has been published in The SMFM Pregnancy Journal — the official open-access journal of the Society for Maternal-Fetal Medicine (SMFM) based in Washington, DC.
Our first peer-reviewed journal article has been published in The SMFM Pregnancy Journal — the official open-access journal of the Society for Maternal-Fetal Medicine (SMFM) based in Washington, DC.
In collaboration with The University of Western Australia, our study demonstrates the capability of our technology, DelivAssure, in detecting early signs of fetal lactic acidosis, highlighted by the strong correlation between sensor current and venous blood lactate levels.
We're proud to contribute to the growing body of evidence aimed at improving outcomes for mothers and babies — DelivAssure has the potential to revolutionise intrapartum care by enabling crucial early detection and management of fetal asphyxia during labor and delivery.
📰 Read the full article here: https://onlinelibrary.wiley.com/doi/10.1002/pmf2.70017
We're incredibly grateful to our collaborators, clinical partners, and the SMFM editorial team for supporting this work.